
In a rapidly evolving clinical research environment, patient engagement is no longer a nice-to-have – it’s a critical success factor. Studies show that up to 30% of patients drop out of clinical trials, often due to poor communication, lack of understanding, or logistical challenges. This dropout not only delays timelines but can also derail trial outcomes entirely. So how can research teams build a better, more patient-centric experience?
This blog explores 11 proven strategies and cutting-edge tools to boost engagement, retention, and satisfaction – right from enrollment to post-trial communication. Whether you’re a sponsor, CRO, or site coordinator, you’ll learn how to implement data-driven, digital-first approaches that meet today’s patient expectations.
In today’s evolving landscape of clinical research, patient engagement has emerged as a pivotal factor influencing the success and efficiency of clinical trials. More than just recruitment and retention, patient engagement encompasses the meaningful involvement of participants throughout the clinical trial journey – from study design and enrollment to follow-up and post-trial communication.
Effective engagement enhances the patient experience, fosters trust, and builds long-term relationships between sponsors, investigators, and participants. When patients feel informed, supported, and valued, they are more likely to adhere to study protocols, share accurate data, and remain committed to the trial. This not only improves data quality and regulatory compliance but also accelerates timelines and reduces overall study costs.
As the industry shifts towards more patient-centric models, embedding engagement strategies into the core of clinical trial design is no longer optional-it is essential for generating meaningful outcomes and advancing medical innovation.
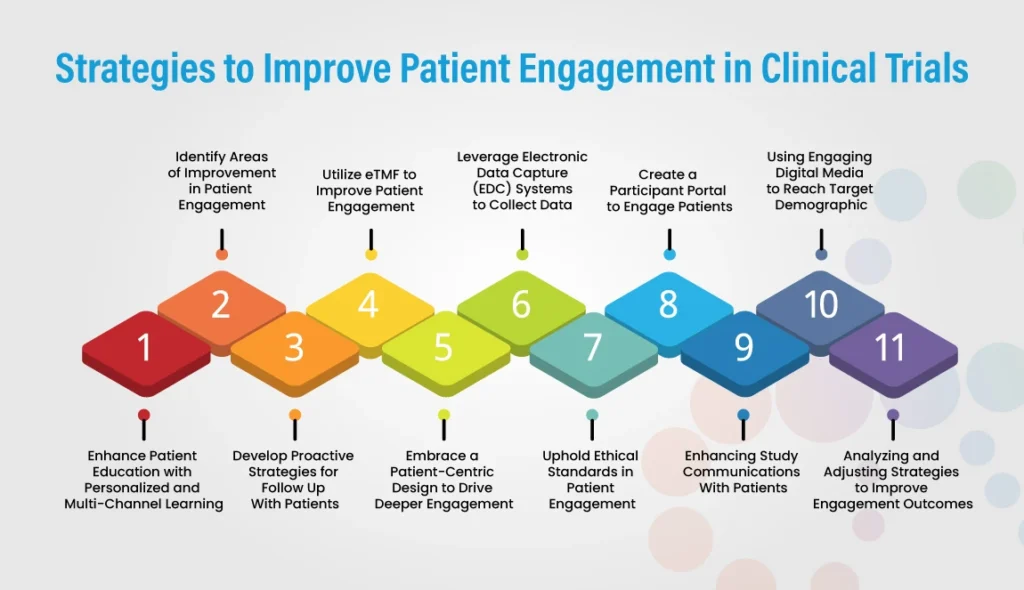
Engaging patients is one of the biggest challenges in conducting clinical trials. Clinical trials need reliable and consistent patient participation. The more engaged and motivated your participants are, the better results you’ll get from your trial – but how can you maximize patient engagement?
There are many different strategies to help improve patient engagement, from the very start of a trial to its conclusion. From identifying key stakeholders to utilizing digital platforms, these eight steps will help ensure your patient engagement levels remain high throughout your study.
1. Identify Areas of Improvement in Patient Engagement
Clinical trials are essential for research and progress, but keeping patients engaged and informed throughout the process is key to success. eTMF (electronic trial master file) is a tool that allows for efficient storage and tracking of all trial documents to streamline the clinical trial process.
Using eTMF helps to ensure that patient engagement remains high by providing quick access to all relevant documents, from protocol summaries to patient information sheets. It also allows sponsors and sites to easily review and manage documents, which reduces the burden on staff who would otherwise be responsible for manually tracking these documents.
One centralized repository ensures that all information is up-to-date, as any changes can be immediately reflected in the eTMF system. This helps to minimize any confusion or inaccuracies during a clinical trial, allowing patients to trust that they are receiving accurate information throughout the process.
Electronic Data Capture (EDC) system is an effective tool for collecting data in clinical trials. Here are some ways that EDC systems can be leveraged to collect data:
Overall, EDC systems are an effective tool for collecting data in clinical trials. By improving accuracy, efficiency, accessibility, security, customization, and data monitoring, EDC systems can help ensure that the data collected is of high quality and can be used to inform clinical decision-making.
Creating a participant portal is an effective way to engage patients in clinical trials. A participant portal is a website or mobile application that allows patients to access information about the trial, communicate with study coordinators, and participate in the trial remotely. Here are some ways a participant portal can be used to engage patients:
Overall, creating a participant portal is an effective way to engage patients in clinical trials. By providing easy access to trial information, facilitating communication, sending reminders and notifications, remote monitoring, and gamification, a participant portal can help improve patient engagement and adherence, leading to more accurate and reliable trial results.
Engaging digital media is an effective way to reach the target demographic and engage patients in clinical trials. Digital media includes social media, online ads, blogs, videos, and other types of online content. Here are some ways digital media can be used to reach the target demographic:
Ultimately, a patient-centric approach reframes the clinical trial as a partnership rather than a protocol. By embedding empathy, flexibility, and transparency into every layer of the trial design, sponsors can foster stronger relationships and improve both short-term engagement and long-term participation rates.
Engaging patients in clinical trials is about more than just recruiting them. It’s also about keeping them engaged throughout the trial. Developing proactive strategies for follow-up with patients can help ensure that they stay with the trial until completion.
Here are some strategies that can help you improve patient engagement and adherence to the protocol:
Before participation begins, every patient must be provided with clear, comprehensive, and understandable information about the trial. This includes:
To enhance the consent process:
Patients must be assured that their personal and medical information is:
How to implement:
Participants should be treated as partners in research, not as passive subjects. This includes:
Why It Matters Embedding these ethical principles throughout the patient engagement journey:
You can never have too much communication regarding patient engagement in clinical trials. Enhancing study communications with patients will help keep them informed of developments and new study details as they arise, which, in turn, can help prevent any potential delays in the study.
Here are some ways you can improve communications with patients: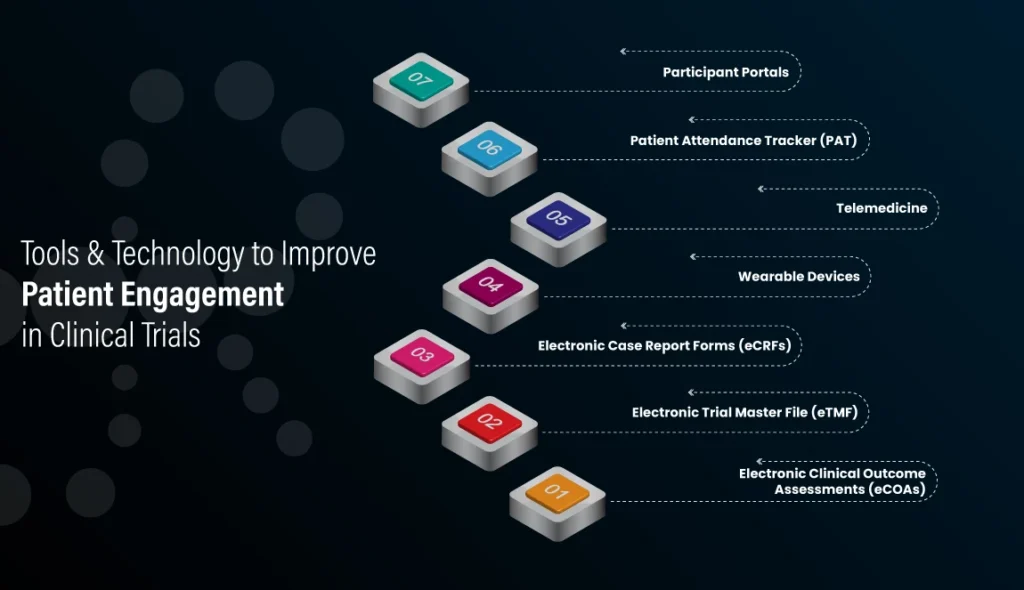
Engaging patients in clinical trials is critical for success. Modern tools and technologies are making it easier to ensure patient participation, satisfaction, and retention. This article explores key digital solutions and best practices to enhance engagement throughout the clinical research lifecycle. Below are the digital patient engagement platform
How eCRFs Enhance Patient Engagement
Improving patient engagement is no longer optional—it’s essential to trial efficiency, compliance, and success. By combining thoughtful strategies like personalized education, digital tools like ePROs and wearable devices, and a truly patient-centric mindset, research teams can build a seamless and supportive experience that patients want to be part of.
When patients are informed, empowered, and respected, engagement becomes natural – and your trial becomes faster, more compliant, and more successful.

Clinvigilant Research is a global full-service CRO (Contract Research Organization) dedicated to advancing clinical development with scientific precision and patient-centric solutions. With our end-to-end CRO services sponsors can accelerate time-to-market while maintaining the highest standards of quality and compliance. With global reach and deep therapeutic expertise, we partners with sponsors to bring safe, effective, and innovative therapies to patients worldwide.
| Cookie | Duration | Description |
|---|---|---|
| _wpfuuid | 11 years | This cookie is used by the WPForms WordPress plugin. The cookie is used to allows the paid version of the plugin to connect entries by the same user and is used for some additional features like the Form Abandonment addon. |
| cookielawinfo-checkbox-advertisement | 1 year | Set by the GDPR Cookie Consent plugin, this cookie is used to record the user consent for the cookies in the "Advertisement" category . |
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| CookieLawInfoConsent | 1 year | Records the default button state of the corresponding category & the status of CCPA. It works only in coordination with the primary cookie. |
| elementor | never | This cookie is used by the website's WordPress theme. It allows the website owner to implement or change the website's content in real-time. |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |
| Cookie | Duration | Description |
|---|---|---|
| GetLocalTimeZone | session | No description |