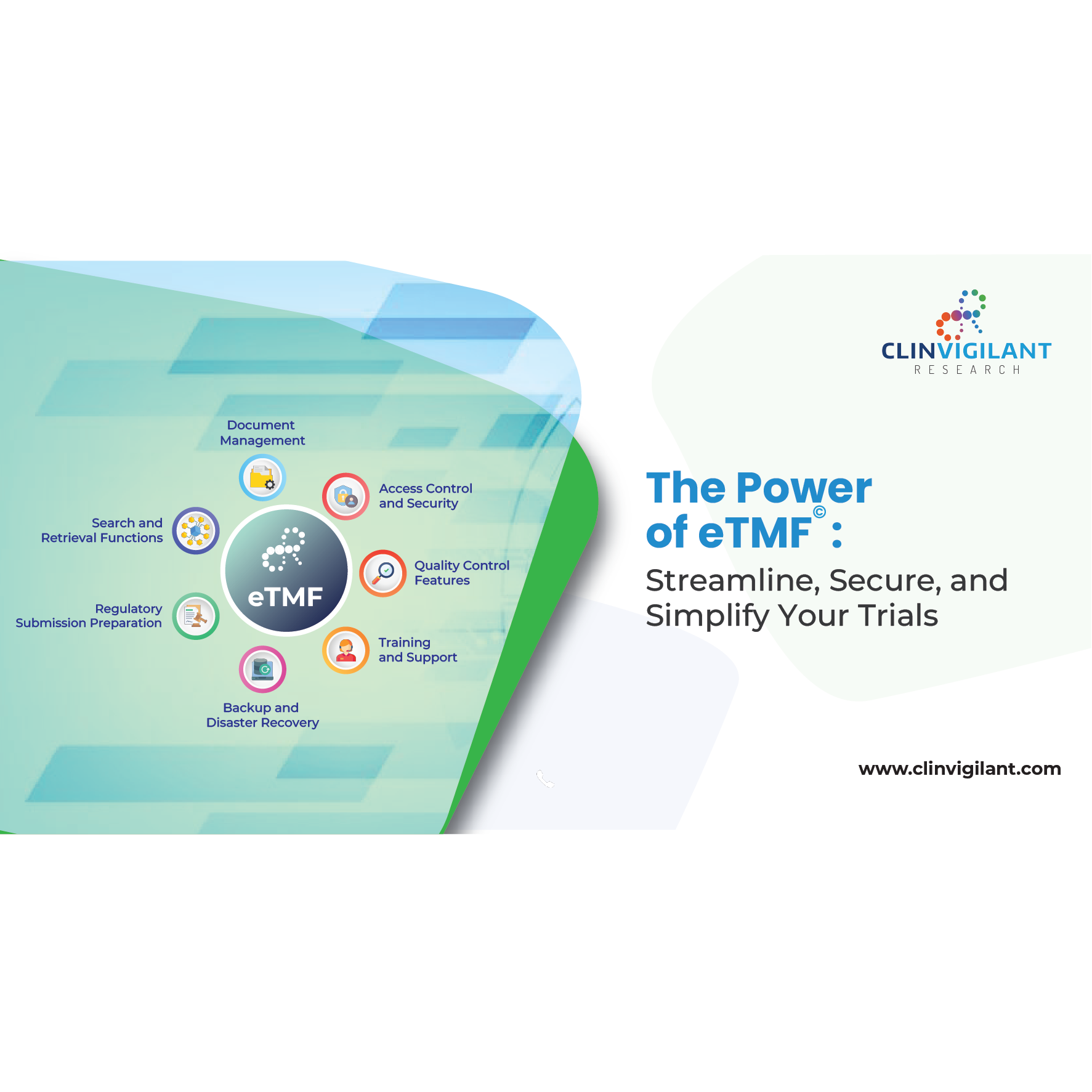
Clinical Data Management: Process, Benefits, and Solutions to Key Challenges
In the complex and highly regulated landscape of clinical research, ensuring data quality, accuracy, and regulatory compliance is essential. Clinical Data Management (CDM) plays a pivotal role in the systematic collection,