
In today’s evolving clinical research landscape, patient engagement is no longer optional – it’s essential. As trials become more complex and patient populations more diverse, involving patients meaningfully throughout the research process has proven to enhance everything from study design and enrollment to retention, data quality, and public trust.
In this blog, we’ll explore why patient engagement matters more than ever, the measurable impact it delivers, the barriers that sponsors, sites, and CROs often face, and the solutions and future innovations shaping a more patient-centered research environment.
Patient engagement in clinical trials refers to involving patients in the research process to ensure their needs and perspectives are taken into account.
This can include activities such as educating patients about the clinical trial process, involving them in the design of the trial, and obtaining their feedback throughout the study.
Patient engagement can help improve clinical trial outcomes by increasing enrollment and retention rates, reducing study dropout rates, and improving patient satisfaction.
Sponsors, sites, and CRO increasingly ask: Why is patient engagement important in clinical research? The answer lies in its ability to deliver measurable benefits-improving study relevance, accelerating enrollment, boosting retention, and enhancing data quality across the board.
Let’s take a closer look at these benefits.
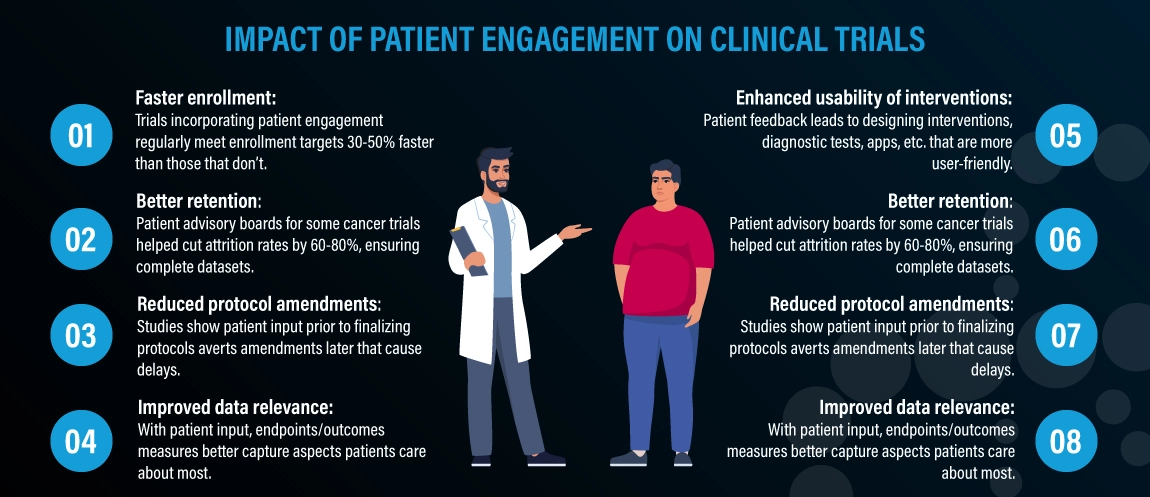
What impact does patient engagement have on the concrete results and outcomes of clinical trials? Here are some of the top findings:
There are various technical methods and tools for enabling patient engagement in clinical trials:
Implement targeted recruitment strategies using data-driven patient profiling to ensure diverse and representative participation.
Provide tailored education and support to empower all patients to engage meaningfully in the trial process.
Clearly define patient roles and expectations from the outset to ensure meaningful engagement and collaboration.
Implement flexible scheduling, remote participation, and support services to accommodate diverse patient commitments..
Privacy Concerns
Patients asked to share study experiences publicly may be uncomfortable or have employer concerns
Ensure voluntary participation and offer anonymous or private sharing options to protect patient comfort and employment concerns.
Develop clear protocols and training to address privacy, safety reporting, and compliance when involving patients in research.
Advocate for dedicated engagement budgets in research grants and integrate patient involvement into project planning from the start.

Clinvigilant Research is a global full-service CRO (Contract Research Organization) dedicated to advancing clinical development with scientific precision and patient-centric solutions. With our end-to-end CRO services sponsors can accelerate time-to-market while maintaining the highest standards of quality and compliance. With global reach and deep therapeutic expertise, we partners with sponsors to bring safe, effective, and innovative therapies to patients worldwide.
| Cookie | Duration | Description |
|---|---|---|
| _wpfuuid | 11 years | This cookie is used by the WPForms WordPress plugin. The cookie is used to allows the paid version of the plugin to connect entries by the same user and is used for some additional features like the Form Abandonment addon. |
| cookielawinfo-checkbox-advertisement | 1 year | Set by the GDPR Cookie Consent plugin, this cookie is used to record the user consent for the cookies in the "Advertisement" category . |
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| CookieLawInfoConsent | 1 year | Records the default button state of the corresponding category & the status of CCPA. It works only in coordination with the primary cookie. |
| elementor | never | This cookie is used by the website's WordPress theme. It allows the website owner to implement or change the website's content in real-time. |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |
| Cookie | Duration | Description |
|---|---|---|
| GetLocalTimeZone | session | No description |